american academy of pediatrics covid vaccine under 12
NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID-19 vaccines for children under 12. Ensure staff is equipped and trained to respond to possible severe allergic reactions in young children.

Us Officials Set Stage For Vaccination Campaign For Younger Children Coronavirus The Guardian
And to date more.

. The FDA issued an EUA for the Pfizer vaccine for kids aged 5 to 11 on 10292021 followed by ACIP recommendation and CDC approval on 1122021. Food. Because theyre still growing and developing its critical that these vaccines are evaluated in well-designed and well-conducted clinical trials Children under 5 years would receive doses of 3 micrograms µg.
ITASCA IL -- Today the Food and Drug Administration FDA finalized the full approval of the Pfizer-BioNTech COVID-19 vaccine for ages 16 and older. The American Academy of Pediatrics AAP in collaboration with the Centers for. Children and COVID Vaccine Data Report.
WHEREAS COVID-19 vaccines are safe and effective. Judy there are 48 million kids in America under the age of 12 but the timeline for a vaccine for those kids keeps changing. 2 days agoThe number of new COVID-19 cases among children in the US.
Update immunization information systems to allow for younger ages groups. It is a lower dose than the 10-µg doses used for children ages 5-11 years and 30-µg doses used for those 12 years and older. Updated weekly this data report tracks the progress in vaccinating US children under 18 years of age.
AAP calls for completion of clinical trials of the vaccine in younger ages to identify correct dose. Grew nearly 76 last week from two weeks prior the American Academy of Pediatrics said MondayAccording to the latest report from the. He serves as founding dean of the National School of Tropical Medicine Professor of Pediatrics and Molecular Virology Microbiology at Baylor College of Medicine where he is also Director of.
Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible. FDA authorizes COVID-19 vaccine for adolescents ages 12-15. The American Academy of Pediatrics AAP is urging the US.
AAP CDC recommend COVID-19 vaccine for ages 12 and older May 12 2021 In addition to approving the Pfizer-BioNTech vaccine for adolescents the CDC updated its clinical guidance to allow coadministration of vaccines. Neither the Johnson Johnson or Moderna vaccine have been approved for anyone under age 18. The FDA gave the Pfizer-BioNTech COVID-19 vaccine emergency authorization to use in children ages 5-15 years old and full approval to use in people ages 16 years and older.
Children ages 5 through 11 years are eligible to receive the Pfizer-BioNTech COVID-19 vaccine. Consider offering COVID flu and other routine vaccines to family members. Face Masks and Other Prevention Strategies.
Learn how it safely teaches your childs immune system to recognize and fight the coronavirus that causes COVID-19. The best way to slow the emergence of COVID variants is to get vaccinated. AAP guidance developed in support of collaborative decision-making.
Parenting a child under 12 can be maddening and scary during normal times but the delta variant and an increase in pediatric covid cases has taken things to a new level. COVID-19 vaccines were evaluated in clinical trials involving tens of thousands of participants and met the US. COVID Cases in Children Data Report.
Vaccine manufacturers are conducting clinical trials to assess the safety and efficacy of COVID-19 vaccination among children younger than 5 years old. Updated weekly find the latest data on COVID-19 cases in children by state. As Covid cases among children continue to rise in the US due to spread of the Delta variant experts are urging the federal government to fast-track vaccine approval for those under the age of 12.
COVID-19 Guidance for Safe Schools and Promotion of In-Person Learning. Only the Pfizer-BioNTech Covid vaccine has been authorized for adolescents and teens ages 12-17. Be prepared to recommend and co-administer influenza vaccine and other childhood vaccines.
Recent research suggests that the Pfizer-BioNTech COVID-19 vaccine authorized for emergency use in children ages 5 to 11 isnt nearly as effective as it is for adolescents or adults. While questions may now arise about the administration of the vaccine off-label for children aged 11 and younger. The AAP recommends COVID-19 vaccination for all children and adolescents 5 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age.
In a New. Food and Drug Administration FDA to approve the use of COVID-19 vaccines in children younger than age 12. On May 12 2021 CDC approved the use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 12-15 years following the vaccines EUA granted by the FDA on May 10th.
Learn more about the process of developing authorizing and approving COVID-19 vaccines. Covid-19The American Academy of Pediatrics answers questions parents may have about the COVID-19 vaccine. Once completed manufacturers submit.
Peter Jay Hotez born May 5 1958 is an American scientist pediatrician and advocate in the fields of global health vaccinology and neglected tropical disease control. The benefits of COVID-19 vaccination outweigh the known and potential risks. Myopericarditis is a severe adverse event to COVID-19 vaccines1 The highest risk has been reported in males aged 12-29 years2-4 According to the US Vaccine Adverse Event Reporting System VAERS the risk in children aged 5-11 years is low with 11 reported cases following 87 million doses of Pfizer-BioNTech vaccine5 However these numbers may be.
While the Covid vaccines in use in. COVID-19 Vaccination Recommendations for Children Under 12. All COVID-19 vaccines approved andor granted Emergency Use Authorization EUA by the Food and Drug Administration FDA and vaccines on the Recommended Child and Adolescent Immunization Schedule including co-administered vaccines are monitored through robust FDA and CDC systems that monitor vaccine safety in the United States.
A single booster dose of the Pfizer vaccine is authorized for kids 12 through 17 years old at least 5 months after. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents. Currently only the Pfizer COVID-19 vaccine has an emergency use authorization for children 12 and up.
Guidance for pediatricians when counseling families about the universal use of face masks by children 2 years of age and older and the adults with whom they interact. This vaccine safety system.
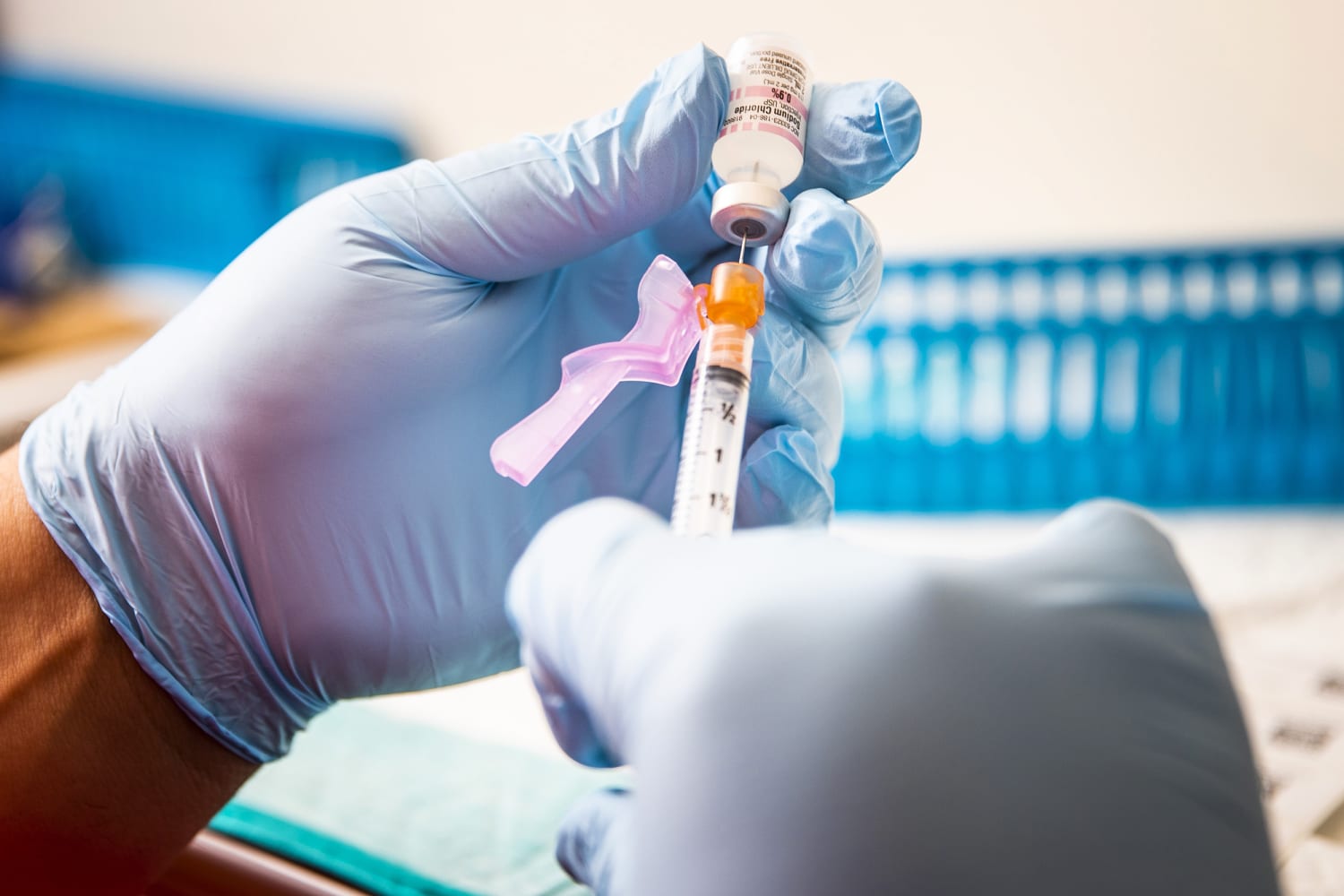
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says
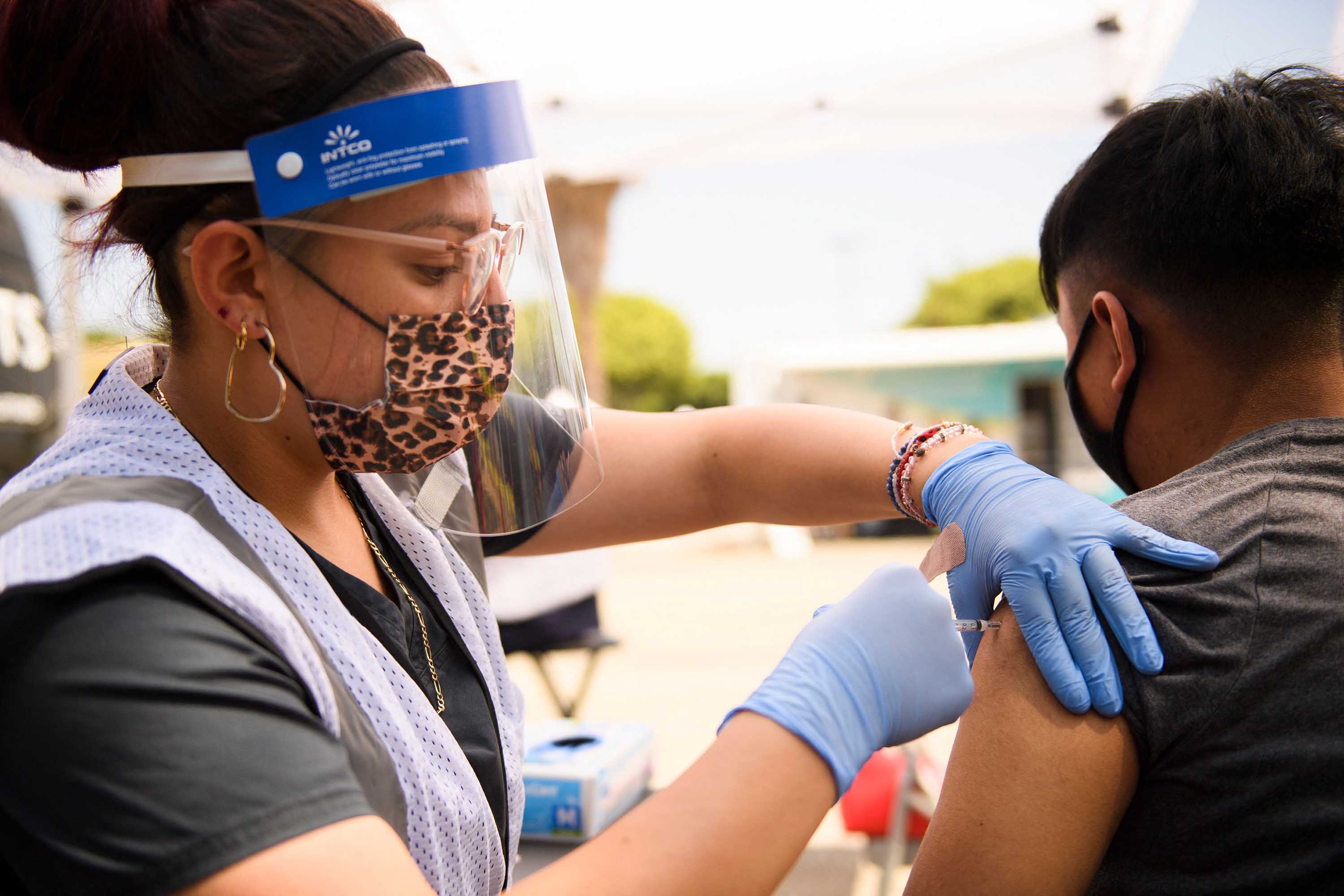
Pediatrician What I Want This Covid Vaccine To Do For My Grandchildren Cnn
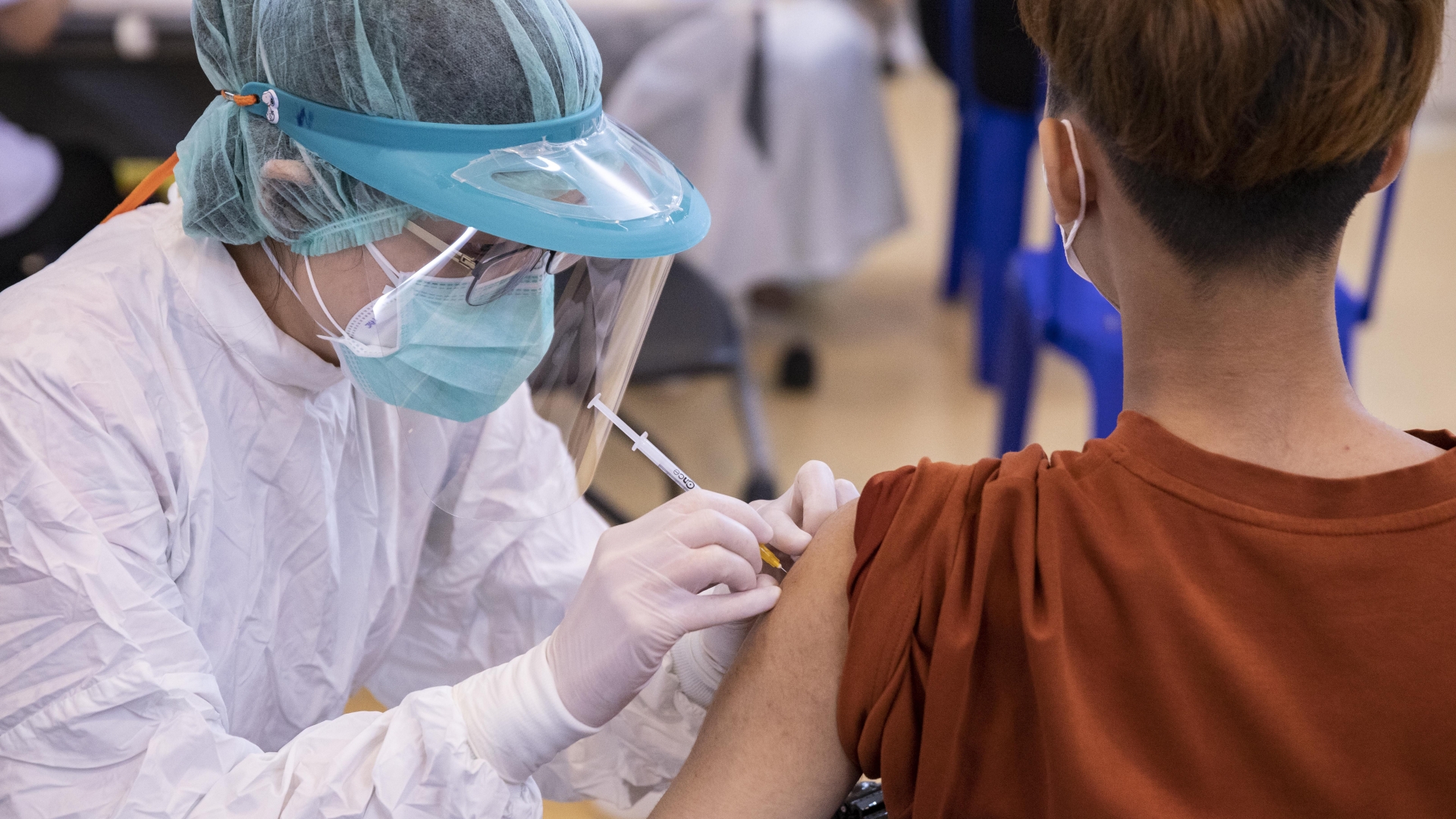
Pfizer Biontech Ask Fda To Authorize Coronavirus Vaccine For Children 5 To 11 The Washington Post
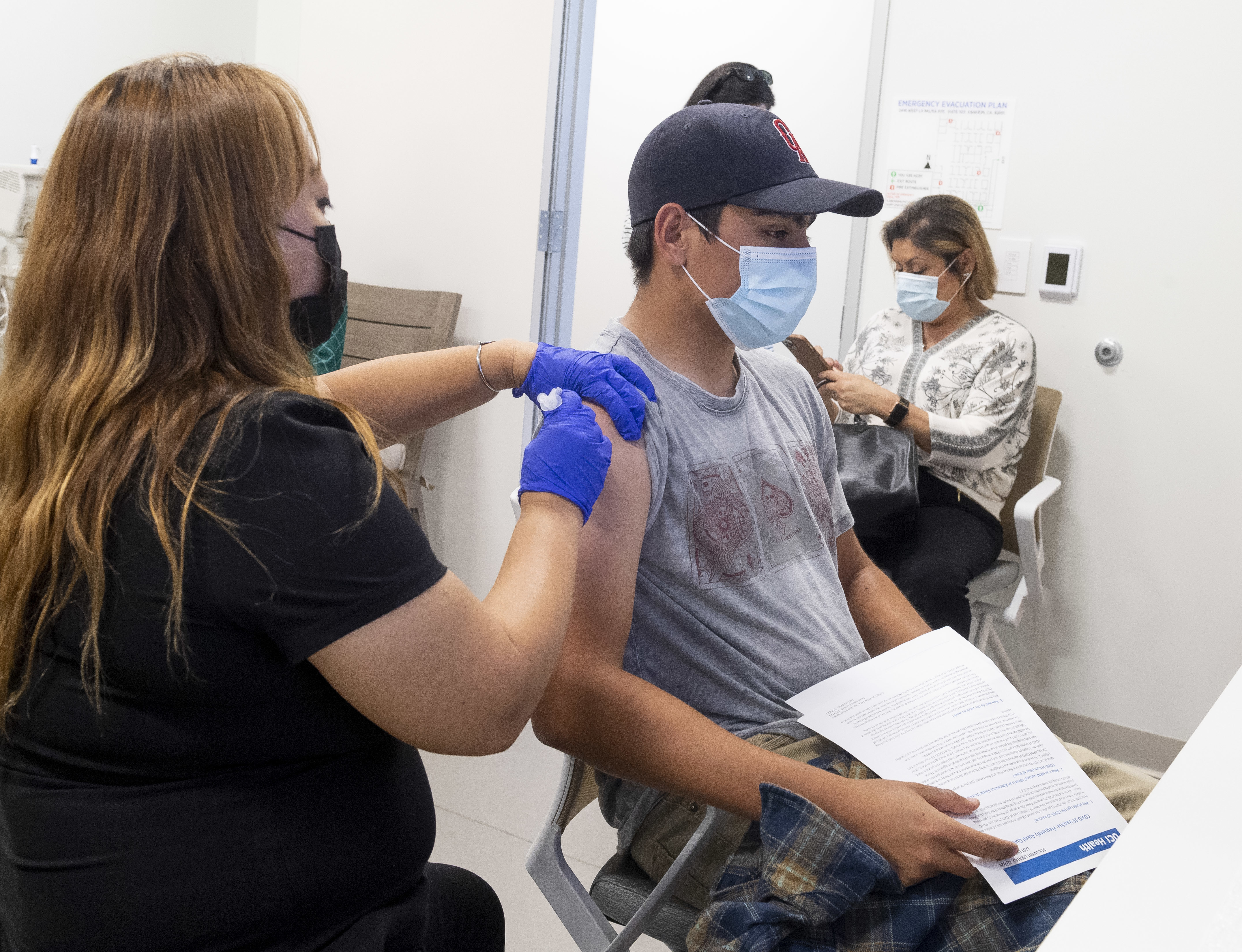
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr
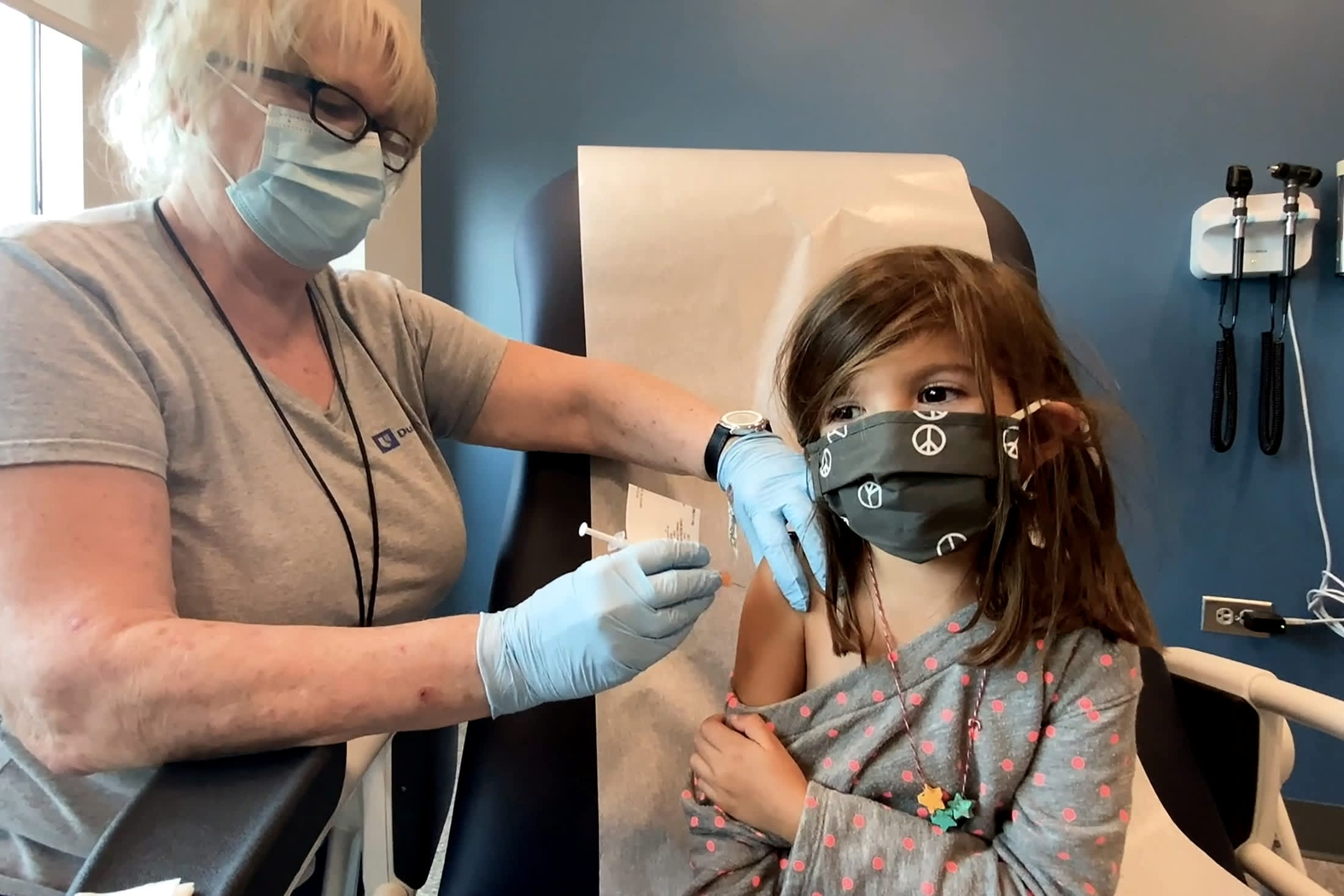
White House Calls On Pediatricians To Help With Rollout Of Kids Covid Vaccine

Us Coronanvirus There Was A 28 Increase In Child Covid 19 Cases Over The Last Two Weeks The American Academy Of Pediatrics Says Cnn
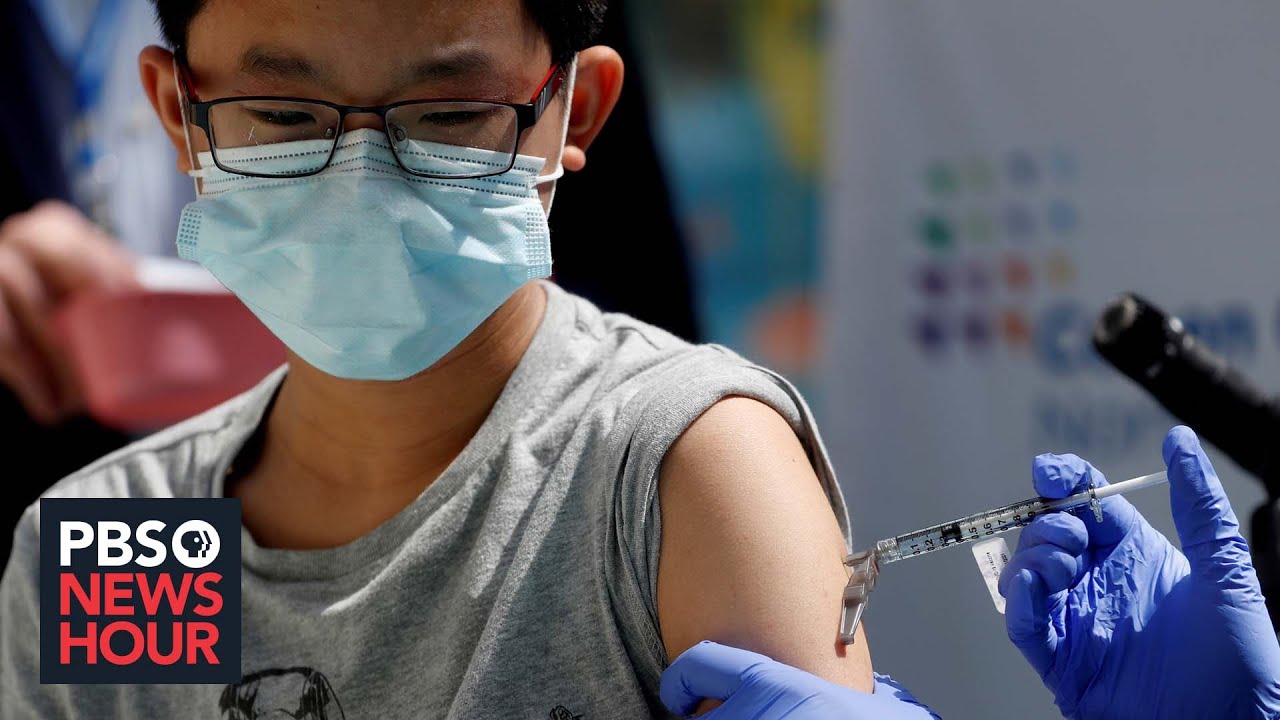
American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube
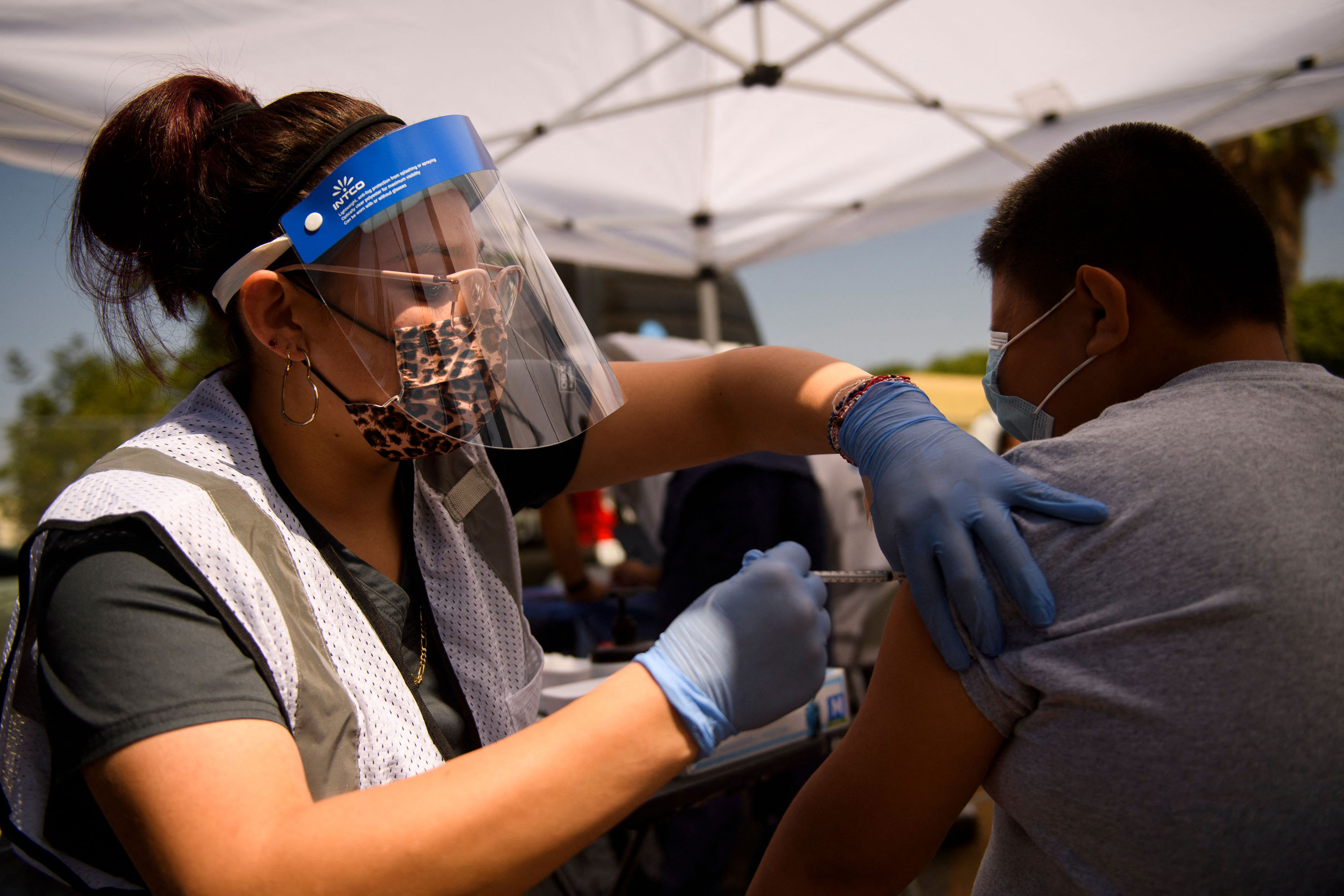
Parents And Pediatricians Are Growing Impatient For A Covid 19 Vaccine For Younger Children Cnn

Pediatricians Plead With Fda To Move Quickly On Covid Vaccine For Kids

What To Know About Children 5 11 Getting The Covid Vaccine The Washington Post

Will Vaccinating Kids Under 12 Get The U S To Herd Immunity
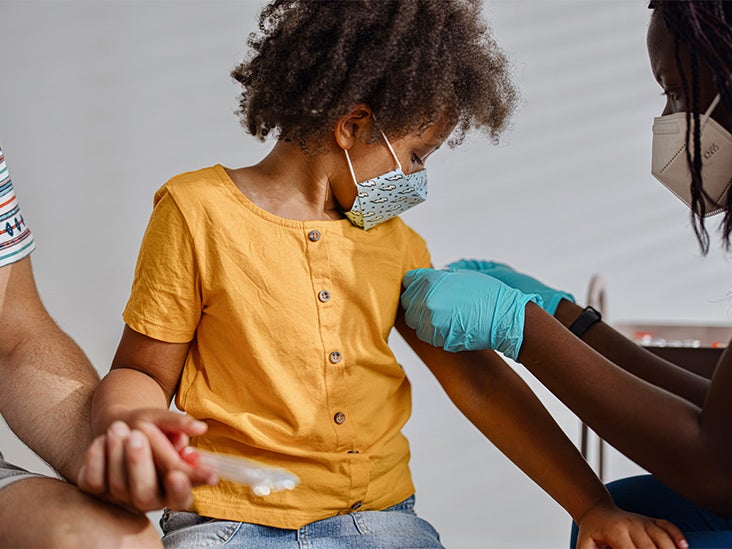
What Parents Should Know About Covid 19 Vaccines For Kids Under 12

More Children Are Catching Covid 19 But It S Unclear If They Re Getting Sicker Coronavirus Updates Npr
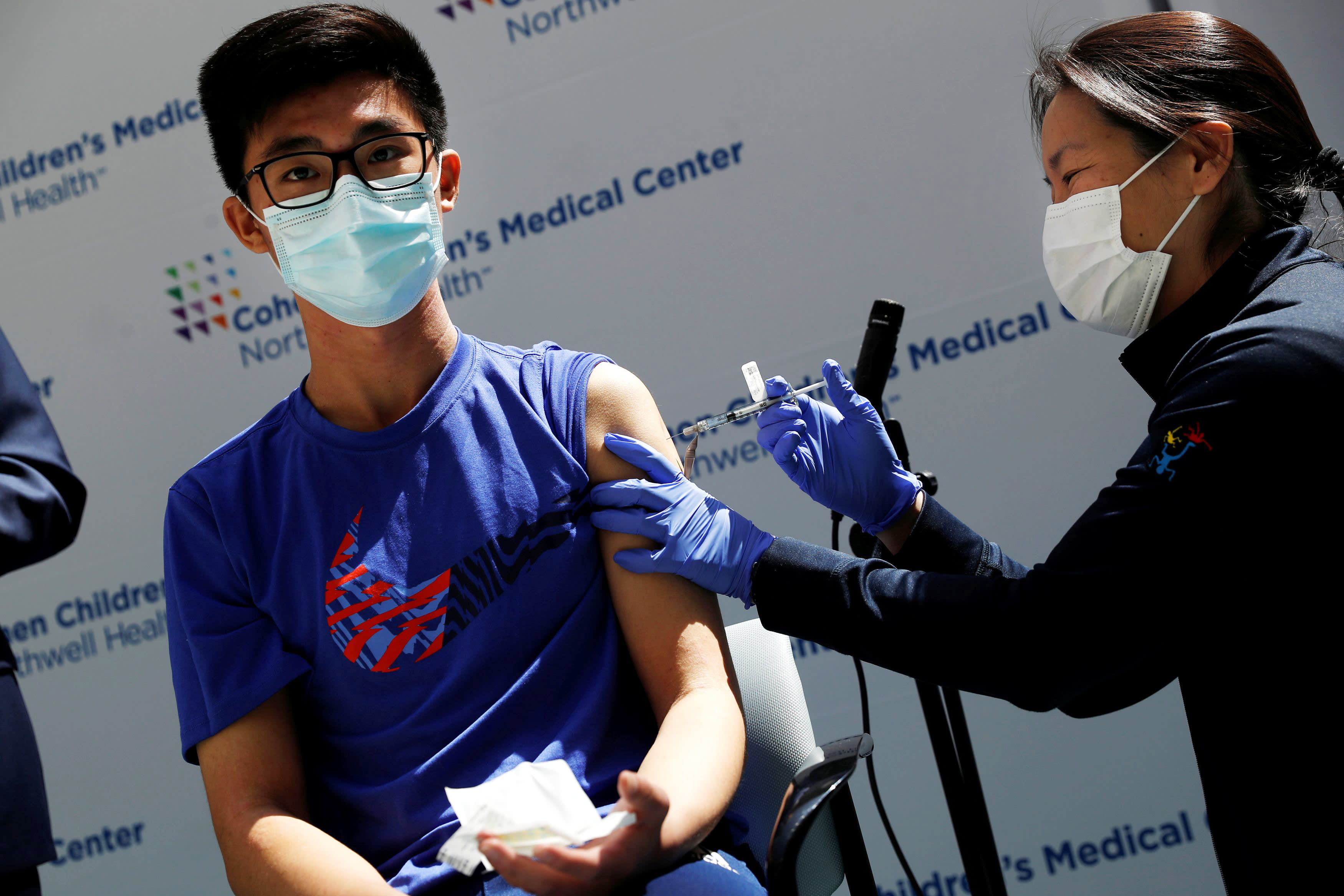
Back To School White House Pushes For Kids 12 And Up To Get Covid Vaccine
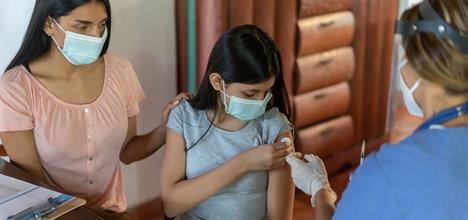
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org
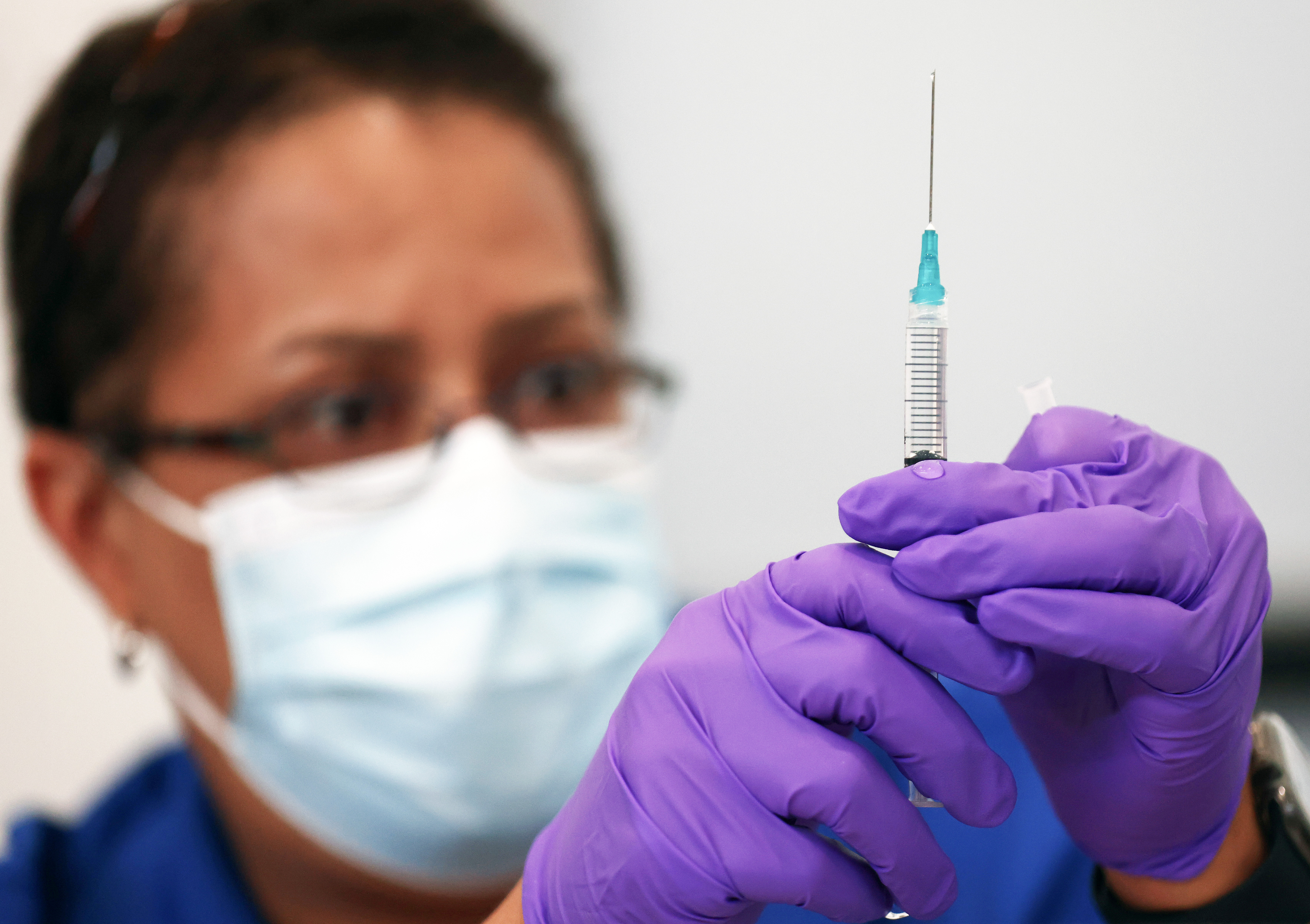
Pediatricians Besieged By Parents Seeking Coronavirus Shots For Kids Under 12 The Washington Post

Some Parents Push To Give Covid 19 Vaccine To Children Under 12 Against Government Guidance Mint

Survey Finds U S Parents Split On Covid Vaccination For Kids Under 12 Consumer Health News Healthday

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News